
The recent announcement by Tesla of Powerwall, its new lithium-ion-based residential battery storage system, has caused quite a stir. It even raises the possibility of going off the grid, relying upon solar panels to generate electricity, and storing it with their own battery and using it on demand.
Yet the lithium-ion technology used by Tesla isn’t the only one on offer. In fact, each of the various battery technologies has its own strengths and weaknesses, and some might even be superior to lithium-ion for home installations. What follows is a quick survey of current battery technologies, and some that are in development.
All rechargeable batteries consist of two electrodes separated by an electrolyte. Two different reversible chemical reactions occur at the two electrodes. While charging, an “active species” — a charged molecule, such as lithium ions for Li-ion batteries — is stored in the anode. During discharge, this migrates to the cathode. The chemical reaction occurs at a potential which can be used to power an external circuit.
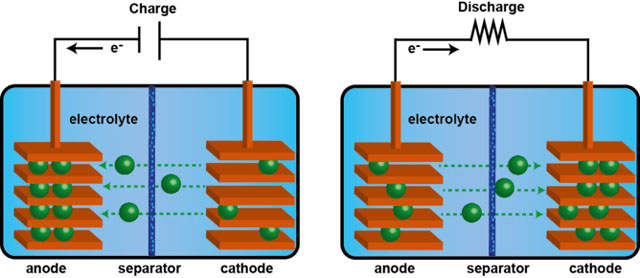
Each type of battery technology can be judged on a number of criteria, such as:
- Recyclability, which is the number of times it can be charged and discharged
- Energy density, which is a measure of the energy stored per unit mass, measured in Watt-hours (a measure representing a Watt of power output over an hour) per kilogram (Wh/kg)
- Specific density, which is the energy stored per unit volume, measured in Watt-hours per litre (Wh/l).
Which technology is best suited for a particular application depends on the demands of that role.
Lead-acid batteries
The original rechargeable battery consists of concentrated sulphuric acid as the electrolyte (H₂SO₄), and lead (Pb) and lead dioxide (PbO₂) on both the anode and cathode, which are both converted to lead sulphate during charge and discharge.
Lead-acid batteries are still used in automobiles, caravans and in some electric relay grids. They have very high recyclability, thus a long lifetime. This is helped by short duration use and constant charging — always keeping the battery at nearly 100% charge — such as occurs in an automobiles. Conversely, slow charge and discharge significantly reduces lead-acid battery lifetime.
Although lead is toxic and sulphuric acid is corrosive, the battery is very robust and rarely presents a hazard to the user. However, if used in a residential installation, the greater size and volume of materials required will also increase the hazards.
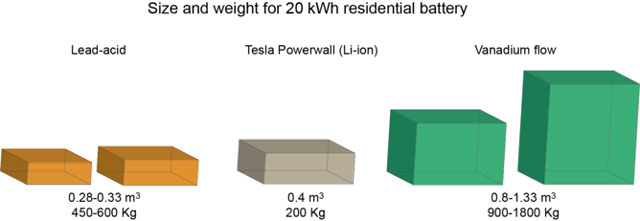
The Li-ion Tesla Powerwall comes in 7kWh or 10kWh versions. For the sake of comparison, we’ll look at what size battery would be required to power a four-person household that consumes 20kWh per day, which is approximately the national average for such homes.
Lead-acid batteries have energy density of 30 to 40Wh/kg and 60 to 70Wh/l. This means a 20kWh system will weigh 450 to 600kg and take up 0,28 to 0,33 cubic metres of space (not including the size or weight of the cell casing and other equipment). This volume is manageable for most households — it would roughly fit in a box 1x1x0,3m — but the weight will mean that it must be stationary.
Lithium-ion
The current premier rechargeable battery is based upon the movement of lithium ions between a porous carbon anode and a Li-metal oxide cathode. The composition of the cathode has a large effect on the performance and stability of the battery.
Currently, lithium-cobalt-oxide exhibits superior charge capacity. However, it is more susceptible to breakdown than alternatives, such as lithium-titante or lithium-iron-phosphate, although these have lower charge capacity.
One common cause of faults is swelling of the cathode as Li ions are inserted within its structure along with the plating of the anode with lithium metal, which can become explosive. The chance of a breakdown can be reduced by limiting the charge/discharge rate, but instances of laptop or phone batteries exploding/catching fire are not uncommon.
The lifetime of the battery also depends heavily on the anode, cathode and electrolyte composition. Generally, the lifetimes of Li-ion are superior to lead-acid batteries, with Tesla reporting a lifetime of 15 years, or (5 000 cycles at one cycle per day) for its 10kWh Powerwall, based on a lithium-manganese-cobalt electrode.
The 10kWh Tesla Powerwall weighs 100kg and has dimensions of 1,3×0,86×0,18m. So, an average four-person household will require two units connected in series, coming to a total weight of 200kg and 1,3×1,72×0,18m or 0,4 cubic metres, which is lighter than lead-acid, but takes up more space.
These values equate to 100Wh/kg and 50Wh/l, which are lower than that reported for Li-cobalt oxide batteries (150-250Wh/kg and 250-360Wh/l), but in the range associated with the safer and longer lifetime Li-titanate (90Wh/kg) and Li-iron phosphate (80 to 120Wh/kg).
Future battery technologies might improve these numbers further. Research labs around the world are working toward improving the specific energy, lifetime and safety of lithium-based batteries.
Major areas of research include changing cathode composition, such as the work with lithium-iron-phosphate or lithium-manganese-cobalt, where different ratios or chemical structures of the materials can drastically affect performance.
Altering the electrolyte, such as using organic or ionic liquids, can improve the specific energy, although they can be cost prohibitive and require more controlled fabrication, such as in a dust-free or humidity controlled/restricted environment.
The use of nanomaterials, in the form of nano-sized carbon analogues (graphene and carbon nanotubes) or nanoparticles, might improve both the cathode and the anode. In the anode, highly conductive and strong graphene or carbon nanotubes can replace the current material, which is graphite or a mixture activated porous carbon and graphite.
Graphene and carbon nanotubes exhibit higher surface area, higher conductivity and higher mechanical stability than activated carbon and graphite. The exact composition of most anodes and cathodes are currently a trade secret, but the commercial production levels of carbon nanotubes hint that most phone and laptop batteries currently have carbon nanotubes as part of their electrodes.
Lab-based batteries have shown incredible storage capacity, particularly for specific energy (Wh/kg). But often the materials are expensive or the process is difficult to scale to industrial levels. With further reduction in material cost and further simplification of synthesis, there is no doubt the application of nanomaterials will continue to improve the capacity, lifetime and safety of lithium based batteries.
Lithium-air and lithium-sulphur
Lithium-sulphur and lithium-air batteries are alternate designs with a similar underlying principle of Li-ion movement between two electrodes, with much higher theoretical capacities.
In both cases, the anode is a thin sliver of lithium while the cathode is Li₂O₂ in contact with air in Li-air, and active sulphur in Li-S batteries. Predicted maximum capacities are 320Wh/kg for Li-ion, 500Wh/kg for Li-S and 1 000Wh/kg for Li-air.
The specific energies are related to the lighter weight of lithium on the anode and cathode (replacing graphite/carbon and transition metal oxides) and the high redox potential between the electrodes.
With the anode in these batteries being lithium metal, the large amount of lithium required for a residential scale 20kWh battery pack (18kg for Li-air and 36kg for Li-S) may limit their use to smaller devices in the short-to-medium term.
Sodium-ion and magnesium-ion
Lithium has atomic number of 3 and sits in row 1 of the periodic table. Directly below is sodium (Na, atomic number 11).
Na-ion batteries are considered as viable alternatives to Li-ion, mainly due to the relative abundance of sodium. The cathode consists of Na-metal oxide, such as sodium-iron-phosphate, while the anode is porous carbon. Due to the size of Na ions, graphite cannot be used in the anode and carbon nanomaterials are being researched as anode materials. Additionally, the mass of sodium is greater than lithium, so the charge capacity per unit mass and volume is generally lower.
Magnesium sits to the right of sodium on the periodic table (Mg, atomic number 12) in row 2, which means it can exist in solution as Mg²⁺ (compared to Li¹⁺ and Na¹⁺). With double the charge of Na, Mg is capable of producing twice the electrical energy for a similar volume.
The Mg-ion battery consists of an Mg-sliver anode and an Mg-metal oxide cathode, and has a predicted maximum specific energy of 400Wh/kg. The current research bottleneck is that the double charge on the Mg²⁺ makes it more sluggish in moving through the electrolyte, thus slowing the charge rate.
Flow batteries
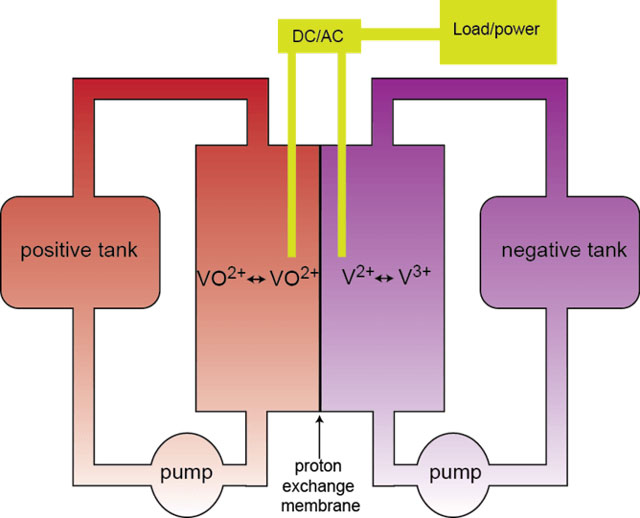
A flow battery consists of two storage tanks filled with electrolyte separated by a proton exchange membrane, which allows the flow of electrons and hydrogen ions, but restricts the mixing of the electrolyte in the storage tanks. Examples of these include vanadium-vanadium with sulphate or bromide, zinc-bromine and bromine-hydrogen.
Vanadium flow batteries have very long lifetimes with the system being very stable. They can be upscaled almost indefinitely but require a pump to cycle the electrolyte around the storage tank. This renders them immobile.
Vanadium flow batteries have specific energies in the range of 10-20Wh/kg and energy density of 15-25Wh/l. That means in order to power a 20kWh household, you’d need a battery with mass of 900-1800kg, which will take up 0,8-1,33m³.
With high reliability but high mass, the vanadium flow cell battery is more suitable for large applications such as small power plants than for residential use.
In the short term, it is likely that Li-ion batteries will continue to be improved, and may even reach 320Wh/kg. Future technologies have the capability to deliver even higher specific energy and/or energy density, but are expected to enter the market first in smaller devices before moving toward residential energy storage.![]()
- Cameron Shearer is research associate in physical sciences at Flinders University
- This article was originally published on The Conversation




